Dr Chloe Ormonde is a lab technician at the BRAIN Unit. In this piece, Chloe shares her career journey so far and why she enjoys working in the laboratory.
About me
I’m Chloe, and I’ve worked for the university for over 16 years. I studied at Cardiff University myself, completing a BSc degree in Pharmacology and a PhD in cardiovascular sciences. I joined the laboratory group of Professor William Gray and the BRAIN Unit at the end of 2016. When I’m not in the lab, I’m a busy mum to two children, a son who is five and a daughter who has just turned 16 months. My time outside of work is normally spent exploring the various playgrounds around Cardiff, building LEGO and practicing ‘first words’ with my daughter. I’m a keen cook, and I enjoy experimenting and trying out new recipes with my family.
My role at the BRAIN Unit
A laboratory technician has the responsibility of overseeing all activities that take place within the research team. On a typical day, we assist in preparing for collection, and the processing of primary human tissue, which is obtained from consented patients undergoing elective neurosurgery at the University Hospital of Wales (UHW), Cardiff. Under the Human Tissue Act, we are responsible for keeping track of all human tissue activities taking place within our research team, and keeping inventories regularly updated.
We also provide technical support for our research group and are responsible for tasks such as ordering and stock checking, protocol generation, formulation of risk assessments and standard operating procedures, as well as providing laboratory support by training new members of staff and students to work safely within the lab and ensuring the correct usage of laboratory equipment.
Why are lab technicians so important?
The technician role is integral to the research team. It is important because we carry out practical and technical support to all staff and students working within the laboratory. We deliver an array of essential, routine laboratory techniques to support any ongoing scientific research projects and are involved in receiving, labelling, and analysing samples. We are responsible for making sure the laboratory remains a user-friendly space, by keeping the lab benches clean and clutter free, disposing of rubbish and keeping track of laboratory consumables such as reagents and plasticware.

What I love about being a lab technician
I’ve always enjoyed tissue culture studies and the fact the BRAIN Unit works directly with human tissue collected straight from surgery really piqued my interest in this role. I was intrigued to see how a 3D representation of normal and diseased brain tissue could be generated in the laboratory. I was also interested in the exciting prospect of using this tissue not only to closer investigate the complex processes that underpin neurological disease, but also as a model to predict the clinical efficacy of drug treatments. These processes help us to identify resistance, toxicity, and aid therapeutic strategies.
With thanks to Chloe for her contributions.
The BRAIN Unit were delighted to support the launch of the new Huntington’s Disease Centre in Wales which took place in Cardiff University’s Hadyn Ellis Building on Wednesday 8 March, 2024.
Huntington’s Disease (HD) is an inherited neurodegenerative disease which causes brain cells to be lost, impacting thinking, movement, behaviour and mental health.
During a warm welcome to the launch event, Professor Anne Rosser explained that the launch of the Huntington’s Disease Centre in Wales marks a new era for HD research. The aim is to bring together researchers across multiple disciplines, institutions and sectors across Wales for collaboration and to promote world leading research into HD.
The local breadth and depth of expertise in HD research from fundamental understanding of disease biology to the clinical testing of new therapies provides a great opportunity to achieve the ultimate goal of finding treatments that can slow down or stop HD progression. The centre also recongises the importance of continuing to provide support to people and families living with HD. Therefore, research into reducing the impact of HD on families, working alongside key patient-focussed organisations also features as a key theme within the centre.
The centre will continue to work alongside its collaborators within Cardiff University, which include the BRAIN Unit, National Neuroscience and Mental Health Innovation Institute (NMHII), the Centre for Neuropsychiatric Genetics and Genomics (CNGG) and the Cardiff University Brain Research Imaging centre (CUBRIC).
The BRAIN Unit looks forward to building on the existing foundation of excellence in HD through collaborative activity.
On Saturday 20 April, BRAIN co-hosted an event with the National Centre for Mental Health (NCMH) to celebrate the importance of patient and public involvement (PPI) in research.
Whilst BRAIN is developing and trialling advanced therapies for neurodegenerative disease and NCMH is looking into the causes of mental health disorders, both are united in one cause: ‘Working together for better brain health’.
“PPI is the most important part of the health research puzzle…”
Public and patient involvement is an integral part of BRAIN and NCMH’s research, as it brings both researchers and members of the public together to help shape and inform the direction of research. This makes research outcomes more reliable, more relevant, and more likely to be used to improve health and social care services.
On the day of the event, BRAIN and NCMH PPI groups had a chance to meet in person and attend a guided lab tour to learn more about how human samples are used in research.
Later on, we welcomed members of the wider public to network with researchers and take part in interactive ‘brain games’. Attendees took part in a variety of discussions from what getting involved in brain health research looks like to an interview with Principal Investigator and BRAIN director, Professor William Gray.
NCMH PPI member, Jacqueline Campbell interviewed Professor Gray on his experiences as a neurosurgeon and working in partnership with PPI groups.

Professor Gray said, “Getting the chance to go into someone’s brain and make a positive difference is such a privilege.”
“It’s important that people who have these conditions have a voice on how these trials are conducted. They are advocates for continuing research in these areas.”
All fun and brain games
Attendees had the opportunity to test and broaden their knowledge of brain health research through an array of interactive stands and brain-related games run by centres from across the Division of Psychological Medicine and Clinical Neurosciences in Cardiff University.
Researchers from NMHII ran two interactive games for guests, including the opportunity to practise their hand at pipetting and guessing different animal brain sizes in a game called ‘Whose brain is it anyway?‘.

Attendees try their hand at pipetting.
Staff based in the Centre for Neuropsychiatric Genetics and Genomics demonstrated how the power of poetry can be used to express experiences of different mental health and brain disorder diagnoses through writing Cinquain poems.
There were also Virtual Reality headset experiences, Pin the ball on the brain, and the ever-popular Stroop Mat.
We also asked guests to take part in our Paint a brain activity which demonstrated how different parts of the brain are responsible for different functions and processes, such as movement and memory.
Soapbox scientists
As well as hearing personal PPI stories, attendees were invited to discover more about specific research areas in the division and pose questions to our ‘soapbox scientists’.
These short talks ranged from the genetics of treatment resistant schizophrenia, the use of virtual reality (VR) in healthcare, and how memory can be impacted by mental illness.
BRAIN Involve lead, Dr Cheney Drew said, “Both BRAIN and NMCH are focused on ensuring that the public and patient voice remains at the heart of the research we carry out. Today’s event enabled us to continue those important conversations between researchers and public contributors. It is important for us to feedback to our public contributors the value they have within the team and the impact they have on research. The event today was a really enjoyable way of being able to do that.”
We’d like to thank all of our attendees for their great insight and contributions on the day.
The BRAIN Unit and National Centre for Mental Health (NCMH) will co-host an event on Saturday 20 April to celebrate the role patient and public involvement groups play in research.
The event will bring together public and patient involvement groups, researchers and the general public who share one common cause: working together for better brain health.
Patient and public involvement (PPI) groups are a vital partnership between members of the public and researchers to make research more relevant, reliable and more likely to be implemented into our health and social care services.
This event will allow attendees to have their say on what public involvement means to them, hear personal stories from BRAIN and NCMH PPI group members, and take part in interactive brain-related activities and panel discussions. We are also offering research lab tours (booking essential),.
This is a free event and we welcome anyone with an interest in brain research and PPI to join us.
Find out more and register your place today.

On Thursday 7 December, our BRAIN Involve lead Dr Cheney Drew delivered a public lecture on the use of advanced therapies in Huntington’s and Parkinson’s Disease.
The lecture was part of the Science in Health public lecture series, hosted by the School of Medicine at Cardiff University. The free public lecture series welcomes a diverse audience, from the public and secondary school pupils to professionals.
In the lecture, Dr Drew spoke about how advanced therapies (ATMPs) can be used as an alternative to traditional drug therapies to treat neurodegenerative disease.
ATMPs fall into three main categories:
- Gene therapy: where a whole or small portion of a gene is inserted into the body
- Cell therapy: where cells are altered to treat and target disease, and
- Tissue engineering: where a combination of cells and tissue scaffolding are modified to enable tissue repair, regeneration or replacement in the body.
Clinical trials in ATMPs such as gene therapy and cell replacement therapy are currently underway to see if they are safe and effective to use in people with neurodegenerative diseases such as Huntington’s and Parkinson’s.
Dr Drew explained exactly how these therapies are used and what clinical trials research currently tells us about their effectiveness, safety and patient experience.

“Clinical trials can be really difficult for people to take part in. It’s really important that we not only monitor for outcome assessment and safety but also understand participant experiences and support their ability to advocate for future therapies.”
Catch up on the lecture and register for the next Science in Health public lecture.
My name is Dr Cheney Drew, and I am a Research Fellow and Senior Clinical Trials Manager based in Centre for Trials Research in Cardiff University.
About me
Outside of my work at Cardiff University, I have many passions, from ultra-marathon running to arts and crafts, as well as baking cakes. On one occasion, I baked a brain cake for Halloween and was reliably informed by neurosurgical colleagues that this was a faithful, and perhaps disturbing representation of the real thing!
My research
I am involved in the design and delivery of high-quality clinical trials, focusing on neurodegenerative and other neurological disorders. This can involves introducing new medicines, or different ways of using existing medicines to non-medicine based ‘interventions’ such as exercise to try and combat disease.
I am also facilitating BRAIN involve, the public and patient involvement group that helps to inform our research activities.
This year, I worked with Dr Emma Lane to understand the experiences of those who take part in complicated clinical trials for degenerative brain conditions, with a focus on Parkinson’s disease.

Why I’m interested in this area of research
There are two big problems we encounter when running a clinical trial: persuading people to take part (recruitment) and making sure that people continue to participate until the end (retention).
These issues make it more difficult to make firm conclusions about the medicine or treatment being tested because there is not enough data to show that the changes to people taking part are because of the treatment and not due to chance.
We hope that by better understanding the participant experience, we can design clinical trials that make it easier and more acceptable for people to take part in and remain, maximising both recruitment and retention and making sure that the clinical trial has the best chance of providing a definite answer about the treatment being studied.
Our findings
We found that taking part in a clinical trial is a big undertaking for the participant, their partners and close family members, though rewarding on the whole.
Participants said that ongoing communication between them and the research team was extremely important, particularly when the trial finished for them but the results are not available for some time.
Also, the transition from being a participant back to a patient can be really difficult for the participant and that needs to be carefully managed, as well as providing participants with payment for travel and accommodation upfront so they are not left out of pocket.
You may think that some of these considerations are not particularly surprising and are often already factored into clinical trial designs. However, our data has demonstrated that small changes in the way this is managed (i.e. paying for those costs upfront and not requiring the participant to apply for reimbursement) would make the experience much better for participants.
Learn more about the LEARN study
We created some short information videos and accompanying documentation that can be used to support people with Parkinson’s disease in taking part in clinical trials, using the data from our study.
As we move away from the challenges of COVID we have been able to harness the benefits of online tools and reimagine our involvement and engagement delivery. In this article, we shine a light on some of the PPI activities from 2022 – 23.
Our Brain Involve group continues to meet online and this has enabled geographical spread across Wales and plans are ongoing for face-to-face activities in the coming year. Our Brain Involve team have informed and supported grant and fellowship applications for senior scientists and early career researchers.
From Brownies and Cubs, to people living with neurodegenerative diseases in the UK and abroad, our face-to-face engagement programme has made a welcome return. One of our biggest events was a full day of activities delivered to over 300 pupils at two local primary schools.
This formed part of the Radyr and Morganstown Festival and had huge support from Headway Cardiff, a brain injury charity. Everyone got involved in learning about the brain, testing their own brains, brain surgery assemblies and having a bounce on the inflatable brain! Year 6 pupils also produced some beautifully creative pictures and poems inspired by our day and the resources created have now been used in other primary schools.
We have also engaged heavily with the patient community. We have become a driving force, ensuring that patient voices are regularly heard at the Network for European CNS Transplantation and Restoration (NECTAR) conference held this year in Athens in 2022, organising sessions that allow patients to share their voice directly with scientists.
Last November, we held a Huntington’s disease patient and family day, bringing the patient, clinical and scientific communities together to share the research that is going on. Our engagement enables us to build trust with our local communities to then create positive involvement, working with people living with neurodegenerative diseases to shape and inform our science.
One example of our involvement activities this year has been focus groups conducted with people living with multiple sclerosis, discussing their thoughts on genetic testing and their prognosis. We have also worked closely with people with Parkinson’s to create resources to support their participation in clinical trials.
We are delighted to have successfully delivered the UniQure Phase I/II gene therapy trial focussing on knocking down Huntington protein production within neurons in Huntington’s Disease, to three patients in Cardiff. This gene therapy is potentially curative or significantly slowing disease progression in this fatal neurodegenerative disease.
Early results are encouraging and if replicated in the ongoing study will represent a major advance in the treatment of this devastating neurological disease.
As well as being a cutting-edge Gene Therapy, its delivery is minimally invasive, with the complete operative procedure taking place within an MRI scanner so that the delivery targeting can be monitored and performed safely in real-time.
This trial will allow people in Wales access to access innovative Advanced Medicinal Therapeutic Medicinal Products (ATMPs), a core objective of Health & Care Research Wales and of our BRAIN Unit. Given the advanced nature of the neurosurgical techniques involved, this trial will be delivered via our Neurosciences Research Unit (NRU) at University Hospital Wales but will be open to participants across Wales and the UK.
We are currently screening an additional three people to continue the trial. Participants in the UK cohort are also referred from University College London (UCL) as we are the only surgical site in the UK with the MRI stereotaxis delivery system.
The images taken during the UniQure trial have allowed us to 3D model the infusion delivery, to investigate how the gene therapy is distributed to further improve therapy delivery and device design in future trials.
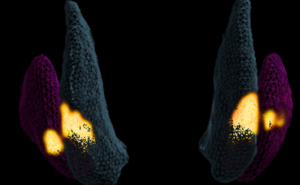
As a Clinical Research Fellow in Neurosurgery, Susruta is working on identifying strategies for optimising the effective delivery of cell and gene-therapies to the brain, under the supervision of Professor Liam Gray.
About me
I am a neurosurgical trainee currently taking time out of my programme to develop my academic training with the BRAIN Unit.
I was keen to return to BRAIN’s excellent environment for translational neurosurgical research, having completed my undergraduate and early-postgraduate medical training at Cardiff University.
The research
Cell and gene-based therapies, collectively known as advanced therapy medicinal products (ATMPs), are expected to revolutionise the treatment of neurological disorders in the upcoming future.

Following decades of pre-clinical investigation, we are at an exciting transition towards ATMPs entering clinical transplantation trials for debilitating neurological disorders such as Huntington’s Disease (HD), Parkinson’s Disease (PD) and temporal lobe epilepsy (TLE). In such diseases, ATMPs must be delivered surgically to specific targets within the brain.
Initially considered to be a trivial problem, achieving effective direct delivery is now recognised as a significant obstacle to treatment success. This is due to the hostile micro-environment arising from a complex combination of surgical delivery, the pre-existing disease, and the patient’s immune response to ATMPs.
Our research is focused on understanding how these inter-related factors can be modified to ensure that ATMP efficacy is maximised. This will require investigation using a series of different models.
Firstly, we will use a transgenic HD model to identify pro-inflammatory cell signalling pathways that are activated after surgical delivery procedures. We can then study the effects of modifying signalling pathways anticipated to have negative effects on ATMP success.
Secondly, we will use an established three-dimensional cell culture model (‘Hi Spot® model’), generated from human brain tissue collected from patients undergoing specific neurosurgical procedures. Comparing ATMP delivery in ‘control’ cortical Hi Spots with sclerotic hippocampal Hi Spots can provide valuable insight into the innate immune response to ATMPs in the inflamed micro-environment.
Research implications
We anticipate that this work will answer crucial questions regarding the ‘science of ATMP delivery’ and pave the way towards more successful treatment of currently incurable neurological disorders in the upcoming era of regenerative neuroscience.
Participants, funders and principal investigators came together to discuss the key findings of a recent Cardiff University study, and to present resources that people with Parkinson’s disease and their support partners can use when considering research participation.
The LEARN study stands for ‘listening to the experiences of participants taking part in neurosurgical trials’. The study sought to understand both the experiences and any barriers to participating in trials involving neurosurgery, to administer both pharmacological and advanced therapies, as well as the the understanding of clinical trials within the general Parkinson’s population.
Researchers obtained this data through interviews with trial participants and an additional survey generated in collaboration with Cure Parkinson’s UK. The interview content was informed by a participant consultation group, ethics advisory group and an independent advisory group made up of neurosurgeons, researchers, and individuals with lived experience of Parkinson’s disease (PD).
From analysis of the interview transcripts, researchers were able to identify key issues affecting the experience and participant’s experience which was then used create informative resources that may help improve the experience of participation in future neurosurgical trials and reduce some of the problems that might cause people to drop out of research.
“The study has highlighted many issues that need resolution before further neurosurgical trials are designed,” said Lesley Gosden, a LEARN study participant.
Key issues identified were challenges associated with brain imaging, taking part in clinical trials when ‘off’ medication and support for support partners, plus the need to understand and retain a lot of information about the trial.
Chief Investigators, Dr Emma Lane and Dr Cheney Drew were pleased to introduce several information videos and written guides on each of these areas so that participants and their carers may be better supported and prepared in future trials.
Support partner, Jayne Calder said, “I believe that the LEARN study has created an opportunity to improve the journey of a participant from start to finish.”
Watch the webinar