BRAIN successfully delivers the UniQure Phase I/II gene therapy trial in Huntington’s Disease
We are delighted to have successfully delivered the UniQure Phase I/II gene therapy trial focussing on knocking down Huntington protein production within neurons in Huntington’s Disease, to three patients in Cardiff. This gene therapy is potentially curative or significantly slowing disease progression in this fatal neurodegenerative disease.
Early results are encouraging and if replicated in the ongoing study will represent a major advance in the treatment of this devastating neurological disease.
As well as being a cutting-edge Gene Therapy, its delivery is minimally invasive, with the complete operative procedure taking place within an MRI scanner so that the delivery targeting can be monitored and performed safely in real-time.
This trial will allow people in Wales access to access innovative Advanced Medicinal Therapeutic Medicinal Products (ATMPs), a core objective of Health & Care Research Wales and of our BRAIN Unit. Given the advanced nature of the neurosurgical techniques involved, this trial will be delivered via our Neurosciences Research Unit (NRU) at University Hospital Wales but will be open to participants across Wales and the UK.
We are currently screening an additional three people to continue the trial. Participants in the UK cohort are also referred from University College London (UCL) as we are the only surgical site in the UK with the MRI stereotaxis delivery system.
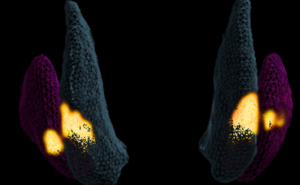
The images taken during the UniQure trial have allowed us to 3D model the infusion delivery, to investigate how the gene therapy is distributed to further improve therapy delivery and device design in future trials.